Nuria Morfin*1,2, & Paul Kozak2 & Collette Mesher3 & Paul Innes2 & Jim Coneybeare4 & Kelsey
Duscharm3 & Ernesto Guzman-Novoa1
1University of Guelph, Honey Bee Research Centre
2Ontario Ministry of Agriculture, Food, and Rural Affairs
3Ontario Beekeepers Association, Technology Transfer Program
4Coneybeare Honey
5nmorfinr@uoguelph.ca
The control of Varroa destructor populations in honey bee colonies is one of the biggest challenges that beekeepers face in Ontario. Beekeepers rely on constant monitoring, the use of cultural or mechanical methods (e.g. removal of drone comb), the use of organic treatments (e.g. formic acid, oxalic acid and thymol), and the use of synthetic acaricides (i.e. tau-fluvalinate/Apistan™, flumethrin/ Bayvarol™, and amitraz/Apivar™) to control V. destructor parasitism (OMAFRA, 2021). The commercial presentation of synthetic acaricides consists of plastic strips impregnated with the chemicals; the strips are placed inside the brood chamber for six to eight weeks for the phoretic mites, which are attached to worker bees, to come in contact with the chemical (Véto-pharma, 2014; Vita beehealth, 2021). Synthetic acaricides have been used for three decades in North America to control V. destructor (Kamler et al., 2016), time during which cases of mite populations resistant to acaricides have been documented (Elzen et al., 1999; Elzen et al., 2000; Rinkevich, 2020).
Resistance to acaricides in V. destructor populations is first noticed by the repeated failure of a product to reach the expected level of control (Coles and Dryden, 2014; Dang et al., 2017). The development of acaricide resistance by V. destructor is a major concern for beekeepers, as high levels of V. destructor in the Fall (i.e. >3%) can translate into high overwinter colony mortality (Guzman-Novoa et al., 2010; OMAFRA, 2021). Thus, a constant surveillance of acaricide resistance is essential to establish informed strategies to control V. destructor, including the correct rotation of different active ingredients synthetic acaricides to treat honey bee colonies, as well as the use of additional methods of mite control (i.e cultural control). Methods to determine acaricide resistance have been developed (Pettis et al., 1998; Rinkevich, 2020; Bahreini et al., 2021). However, even when the tests are designed to detect resistance, they cannot measure the exact level of resistance and also presents logistical challenges like the use of highly parasitized colonies as well time consuming methods often carried out without complete chain of custody. Thus, we conducted preliminary trials to evaluate the use of a glass vial residual bioassay to determine the efficacy of three synthetic acaricides (tau-fluvalinate, flumethrin, and amitraz). The bioassay is aimed at reducing logistical challenges and to have more accurate estimations of resistance levels by determining the lethal concentrations (LCs) and discriminating concentrations (DCs) of acaricides (Elzen et al., 1999; Elzen et al., 2000; Maggi et al., 2008).
Here is what we did:
- Mites were collected from three highly parasitized experimental colonies kept at the Honey Bee Reserch Centre, University of Guelph.
- Dilutions of three chemical grade synthetic acaricides were prepared.
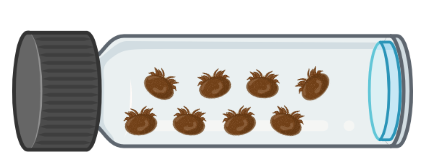
- The walls of glass vials were impregnated with 200 µl containing 0 (control), 0.3, 0.5, 1 and 5 µg of tau-fluvalinate, or flumethrin, or amitraz.
- Once the walls of the glass vials were dried, seven to nine mites were introduced into each vial and left there for four hours. Three repetitions were conducted (with an additional control with no solvent). A total of 54 vials and 443 mites were used for the study.
- After four hours of exposure, the number of dead and live mites were recorded.
- The proportion of dead mites in each treatment was used to analyze and determine a)differences between treatments on their efficacy to kill mites, and b)the concentration at which 50%, 90% and 95% of the mites are killed (LC50, LC90, and LC95).
Testing for synthetic acaricide resistance in mite populations in Ontario is necessary for beekeepers to make informed decisions when designing their Integrated Pest Management strategies in order to achieve adequate control of varroa levels and the survival of their colonies. Based on the results from this study, we will be able to make recommendations on the protocol to test for acaricide resistance under laboratory conditions, by determining discriminating concentrations for each chemical. Also, we will recommend a protocol for conducting trials in collaboration with beekeepers to facilitate mites for testing.
Acknowledgements
This work was supported by the Ontario Animal Health Network (OAHN). The illustrations were created by BioREnder.com. We thank Paul Kelly and Alvaro de la Mora for facilitating highly infested experimental colonies for the study at the Honey Bee Research Centre, University of Guelph.
References.
Bahreini, R., Nasr, M., Docherty, C., Feindel, D., Muirhead, S., & de Herdt, O. (2021). New bioassay cage methodology for in vitro studies on Varroa destructor and Apis mellifera. Plos one, 16(4), e0250594.
Coles, T. B., & Dryden, M. W. (2014). Insecticide/acaricide resistance in fleas and ticks infesting dogs and cats. Parasites & vectors, 7(1), 1-10.
Dang, K., Doggett, S. L., Singham, G. V., & Lee, C. Y. (2017). Insecticide resistance and resistance mechanisms in bed bugs, Cimex spp.(Hemiptera: Cimicidae). Parasites & vectors, 10(1), 1-31.
Elzen, P. J., Eischen, F. A., Baxter, J. R., Elzen, G. W., & Wilson, W. T. (1999). Detection of resistance in US Varroa jacobsoni Oud.(Mesostigmata: Varroidae) to the acaricide fluvalinate. Apidologie, 30(1), 13-17.
Elzen, P. J., Baxter, J. R., Spivak, M., & Wilson, W. T. (2000). Control of Varroa jacobsoni Oud. resistant to fluvalinate and amitraz using coumaphos. Apidologie, 31(3), 437-441.
Guzmán-Novoa, E., Eccles, L., Calvete, Y., Mcgowan, J., Kelly, P. G., & Correa-Benítez, A. (2010). Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie, 41(4), 443-450.
Kamler, M., Nesvorna, M., Stara, J., Erban, T., & Hubert, J. (2016). Comparison of tau-fluvalinate, acrinathrin, and amitraz effects on susceptible and resistant populations of Varroa destructor in a vial test. Experimental and Applied Acarology, 69(1), 1-9.
Maggi, M. D., Ruffinengo, S. R., Gende, L. B., Eguaras, M. J., & Sardella, N. H. (2008). LC50 baseline levels of amitraz, coumaphos, fluvalinate and flumethrin in populations of Varroa destructor from Buenos Aires Province, Argentina. Journal of apicultural research, 47(4), 292-295.
Pettis J.S., Shimanuki H., Feldlaufer M.F. (1998) An assay to detect fluvalinate resistance in Varroa mites. American Bee Journal, 138, 538–541.
Rinkevich, F. D. (2020). Detection of amitraz resistance and reduced treatment efficacy in the Varroa Mite, Varroa destructor, within commercial beekeeping operations. PloS one, 15(1), e0227264.
Ontario Ministry of Agriculture, Food, and Rural Affairs (OMAFRA). Available at http://www.omafra.gov.on.ca/english/food/inspection/bees/apicultu.html [accessed October 4, 2021]
Véto-pharma. Available at https://www.mannlakeltd.com/mm5/graphics/pdfs/Apivar_brochure_USA.pdf [accessed October 4, 2021].
Vita BeeHealth. Available at https://www.vita-europe.com/beehealth/products/apistan/#work [accessed October 4, 2021]