Queen’s Reproductive System
The Reproductive System of the Honey Bee Queen Consists of Paired Ovaries…
Clarence Collison
“The reproductive system of the honey bee queen consists of paired ovaries connected by calices (cuplike cavity or structure) to paired lateral oviducts, which merge to form a median oviduct connected dorsally to the spermatheca and caudally to the terminal portion of the reproductive tract (i.e. genital chamber and bursa copulatrix with lateral pouches). The spermatheca is connected to the median oviduct via the spermathecal duct. Paired spermathecal glands are connected to the spermatheca via the common duct. The calyx is a funnel-like structure on the caudal aspect of the ovaries that facilitates the transfer of eggs from ovarioles to the lateral oviduct. The calyx is composed of simple to the bi-layered tall, columnar, densely packed epithelium along a basement membrane (Kozii et al. 2021).”
“The lateral oviducts extend bilaterally from the calyx and unite ventrally toward the spermatheca, to form the median oviduct. The oviducts are lined by cuboidal epithelium on a basement membrane with a thin, outer layer of longitudinal muscle. The luminal side (central cavity of a tubular structure) of the epithelial lining is covered by a thin, chitinous intima that has numerous cteniform (comb-like) spines pointing caudally. The lining epithelium is attenuated (reduced in thickness) when the oviduct lumen is distended by an egg (Kozii et al. 2021).”
“The median oviduct is a short, muscular tubule that joins both lateral oviducts. It is posteriorly defined by the valve fold, which also constitutes the transition point of the median oviduct into the genital chamber. On the ventral surface, the median oviduct has two lateral folds connected to sphincter muscles. It is lined by epithelium similar to that found in the lateral oviducts, but instead of cteniform spines, it is covered by a thin intima that thickens posteriorly along the genital tract (Kozii et al. 2021).” “The valve fold is a tongue-like structure which can close the passage between the vagina (genital chamber) and the median oviduct of the queen (Eckert and Shaw 1960).” “The valve fold is a deep transverse epithelio-muscular inward projection on the ventral aspect of the posterior median oviduct. The stalk of the valve fold is composed of loosely arranged muscle and is covered by a single layer of cuboidal to columnar epithelium with multiple invaginations. The epithelium of the valve fold is covered by a thin intima, similar to the median oviduct (Kozii et al. 2021).”
“The valve fold in queens is an “oak leaf-shaped” structure, located on the ventral side of the vagina lumen, on the side opposite to the opening of the spermathecal duct. A few muscle fibers are attached to the ventral outer layer of the valve-fold. In previous studies, the function of the organ was presumed to be as follows: 1) assist sperm migration into the spermathecal reservoir (spermatheca) after mating and subsequently prevent sperm loss by closing the genital chamber and 2) arrest of spermatozoa to expose enough spermatozoa into the micropyle of the anterior side of the egg during fertilization (Sasaki and Obara 2002).”
“The spermatheca, spermathecal duct and the spermathecal glands are accessory organs of the reproductive system of the honey bee queen. The spermatheca is located craniodorsally over the median oviduct at the level of the 5th sternum. It is round, 1.2-1.3 mm in diameter, a sperm recepticulum (Tarpy et al. 2011). The spermathecal glands secrete proteins into the spermatheca via the common duct to replace the fluid lost from the spermatheca with sperm during fertilization (Klenk et al. 2004). At the base of the spermathecal duct, the muscular sperm pump provides a constant sperm volume necessary for the fertilization of a single egg (Baer et al. 2016) (Kozii et al. 2021).”
“The spermatheca is a spherical structure containing all of the spermatozoa that will be required during the queen’s life of egg laying. These spermatozoa may need to be stored for several years. The spermatheca is richly supplied with tracheal branches to provide an oxygen supply, and also has two glands, the spermathecal glands, to provide other necessary secretions to maintain the health of the spermatozoa. The two spermathecal glands are long round structures that snake over the surface of the spermatheca. They meet and join the spermathecal duct close to the point where the spermathecal duct has its opening into the spermatheca itself. Around this junction are sets of muscles believed to be responsible for delivering spermatozoa down the spermathecal duct for fertilization (Stell 2012).”
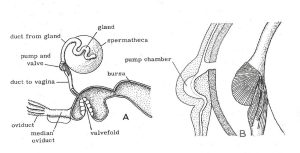
Figure 1
A. The spermatheca and genital chamber (vagina) with
adjoining organs of a honey bee queen.
B. The spermathecal valve and pump in section and external view showing muscles. (Dade 1962)
“The posterior portion of the genital tract comprises the genital chamber, bursa copulatrix, and bursal pouches. During copulation, the chitinous plates of the drone endophallus attach to the bursal pouches (Camargo and Mello 1970; Woyke 2011) and sperm is ejaculated into the lateral oviducts where it then travels backward via the spermathecal duct to be stored in the spermatheca within the next several hours. The valve fold is thought to regulate the backflow of sperm into the spermatheca by preventing its retrograde flow into the posterior genital chamber (Laidlaw 1944). However, since sperm consistently escape backward into the bursa copulatrix after mating, the efficacy of regulation of sperm flow by the valve fold has been questioned by Camargo and Mello 1970 (Kozii et al. 2021).”
“The total volume of semen acquired by a queen during her visit to a drone congregation area greatly exceeds what she will store for use throughout the rest of her life. Each drone injects about 11 million sperm into a queen and the total number of sperm received on a mating flight is some 87 million. Yet a queen typically stores only about 5 million sperm in her spermatheca. Although it is not clear just how randomly a queen samples from among the 87 million sperm in storing away her five million sperm, the way in which the sperm are processed before being stored suggests that a great deal of mixing of the different drones’ gametes can occur. During mating, the sperm are received into the queen’s lateral oviducts. Upon returning to her nest, the queen forces the sperm into her vagina by muscular contractions. The valve-like fold stops much of the semen from flowing back out, and instead directs the sperm into the spermathecal duct and thence into the spermatheca. The excess semen pushes out past the valve fold and out of the queen in the form of thin threads which are removed by the workers (Seeley 1985).”
“Each ovary consists of densely packed clusters of ovarioles which are thin chains of eggs that widen posterior, representing sequential, linear maturation of the oocytes and trophocytes. Each ovariole has four distinct regions which are: a short terminal filament, a germarium of intermediate length, and a long vitellarium which ends with the ovariole pedicle, all encased within an epithelial sheath. The terminal filament is composed of undifferentiated stem cells embedded between discoid cells; the stem cells have large nuclei and poorly stained cytoplasm. The germarium contains germline cells (cystocytes) clustered into rosette-like structures. In the distal portion of the germarium, the oocytes become arranged into a single row, separated by nurse cell chambers, which constitutes the transition to the vitellarium. Somatic cells are haphazardly arranged in the proximal germarium but form a distinct follicular epithelial lining around an oocyte and its corresponding trophocytes in the vitellarium. In the centro-caudal portion of the ovariole, the ovum is almost completely covered by simple cuboidal somatic epithelium with a central nucleus, densely stippled chromatin, leaving only a narrow opening on the anterior side, the trophic stalk which allows access of nutrients from the trophocytes or nurse cells. There is a distinct germinal vesicle within individual oocytes. At the late stages of vitellogenesis, the epithelium of the ovum is blunted (cuboidal) and subsequently becomes attenuated. As they move posterior along the ovariole, the trophocytes increase in size and eventually undergo degeneration. Small median follicular cells, previously described as intertrophocytic median follicular cells, are occasionally observed in between trophocytes within the nurse chamber (Kozii et al. 2021).”
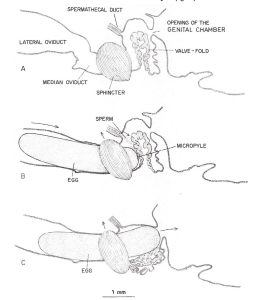
Figure 2
The process of an egg being fertilized within the queen’s median oviduct. The egg micropyle in contact with the valve fold (Camargo and Mello 1970)
“Mature eggs produced from a pair of ovaries are stored in the lateral oviducts until the queen begins an oviposition. The micropyle (a small opening in the egg through which spermatozoa can enter) was observed on the anterior pole of the egg. The micropylar area of an egg consisted of a dense network of canals of various shapes and sizes. The center canals are perpendicular to the margin of the micropylar area, whereas the outer canals generally slant toward the center. The surface of the chorion at the posterior end of an egg was smooth without any radical structure or canals (Sasaki and Obara 2002).”
“Fertilization of an egg takes place in the median oviduct. It is believed that the valve fold, together with the median oviduct sphincter, slow down the egg when it passes through the median oviduct and pushes the micropylar end of the egg against the opening to the spermathecal duct to facilitate fertilization. The valve fold is also thought to regulate sperm outflow from the spermathecal duct during fertilization (Camargo and Mello 1970).”
“Gotoh and Sasaki (2021) histologically examined the developmental process of the internal reproductive organs including spermatheca, valve-fold in the vagina, semi-circular muscle surrounding the common oviduct, and abdominal ganglia in honey bee queens and workers. During the pupal stage, queens showed an increased spermathecal reservoir, development of the tracheal network surrounding the spermathecal reservoir and elongation of the spermathecal gland. Compared with queens, these developmental processes were never observed during the pupal stage in workers. Moreover, development of the valve-fold and semi-circular muscle was aborted, and they became rudimentary at the middle pupal stage in workers. Morphological caste differences in the abdominal ganglia were observed from the prepupal stages, showing that the most posterior ganglion was fused with the anterior ganglia in queens but not in workers.”
“Ratnieks and Keller (1998) investigated the precision with which queens can control the fertilization of the eggs they lay. Because males and workers are reared in different-sized cells, it is possible to know which type of egg a queen “intends” to lay. Eggs were collected from both worker and drone (male) cells from four colonies. Ploidy of the embryo was determined using polymorphic DNA microsatellites. All 169 eggs taken from worker cells were heterozygous at least one microsatellite locus showing that the egg was fertilized. All 129 eggs taken from drone cells gave a single band at the B124 locus, strongly suggesting haploidy. These data show that queens have great, and quite possibly complete, ability to control the fertilization of the eggs they lay.”
“Baer et al. (2016) quantified the number of sperm that queens use to fertilize eggs. They examined sperm use in naturally mated queens of different ages and in queens artificially inseminated with different volumes of semen. They found that queens are remarkably efficient and only use a median of 2 sperm per egg fertilization, with decreasing sperm use in older queens. The number of sperm in storage was always a significant predictor for the number of sperm used per fertilization, indicating that queens use a constant ratio of spermathecal fluid relative to total spermathecal volume of 2.364 x 10-6 to fertilize eggs. This allowed them to calculate a lifetime fecundity for honey bee queens of around 1,500,000 fertilized eggs. Their data provide the first empirical evidence that queens do not manipulate sperm use, and fertilization failures in worker-destined eggs are therefore honest signals that workers can use to time queen replacement, which is crucial for colony performance and fitness.”
References
Baer, B., J. Collins, K. Maalaps and S.P.A. den Boer 2016. Sperm use economy of honeybee (Apis mellifera) queens. Ecol. Evol. 6: 2877-2885.
Camargo, J. and M. Mello 1970. Anatomy and histology of the genital tract, spermatheca, spermathecal duct and glands of Apis mellifica queens (Hymenoptera: Apidae). Apidologie 1: 351-373.
Dade, H.A. 1962. The Anatomy And Dissection Of The Honeybee. Bee Research Association, London, 158 pp.
Eckert, J.E. and F.R. Shaw 1960. Beekeeping. Macmillan Co., New York, 536 pp.
Gotoh, A. and K. Sasaki 2021. Caste differentiation of spermatheca and organs related to sperm use and oviposition in the honeybee, Apis mellifera. Apidologie 52: 262-271.
Klenk, M., G. Koeniger, N. Koeniger and H. Fasold 2004. Proteins in spermathecal gland secretion and spermathecal fluid and the properties of a 29 kDa protein in queens of Apis mellifera. Apidologie, 35: 371–381.
Kozii, I.V., S.C. Wood, R.V. Koziy and E. Simko 2021. Histomorphological description of the reproductive system in mated honey bee queens. J. Apic. Res. https://doi.org/10.1080/00218839.2021.1900636
Laidlaw, H.H. 1944. Artificial insemination of the queen bee (Apis mellifera L.): Morphological basis and results. J. Morphol. 74: 429-465.
Ratnieks, F.L.W. and L. Keller 1998. Queen control of egg fertilization in the honey bee. Behav. Ecol. Sociobiol. 44: 57-61.
Sasaki, K. and Y. Obara 2002. Egg activation and timing of sperm acceptance by an egg in honeybees (Apis mellifera L.). Insectes Soc. 49: 234-240.
Seeley, T.D. 1985. Honeybee Ecology: A Study of Adaptation in Social Life. Princeton University Press, Princeton, NJ.
Stell, I. 2012. Understanding Bee Anatomy: A Full Colour Guide. The Catford Press, Teddington, Middlesex, UK, 203 pp.
Tarpy, D.R., J.J. Keller, J.R. Caren and D.A. Delaney 2011. Experimentally induced variation in the physical reproductive potential and mating success in honey bee queens. Insectes Soc. 58: 569-574.
Woyke, J. 2011. The mating sign of queen bees originates from two drones and the process of multiple mating in honey bees. J. Apic. Res. 50: 272-283.
Clarence Collison is an Emeritus Professor of Entomology and Department Head Emeritus of Entomology and Plant Pathology at Mississippi State University, Mississippi State, MS.










