Wintering Honey Bees
By: Clarence Collison
“In order to survive cold northern winters, honey bees crowd tightly together in a winter cluster. Present models of winter cluster thermoregulation consider the insulation by the tightly packed mantle bees as the decisive factor for survival at low temperatures, mostly ignoring the possibility of endothermic heat production. Stabentheiner et al. (2003) provided direct evidence of endothermic heat production by ‘shivering’ thermogenesis. The abundance of endothermic bees is highest in the core and decreases towards the surface. This shows that core bees play an active role in thermal control of winter clusters. They concluded that regulation of both the insulation by the mantle bees and endothermic heat production by the inner bees is necessary to achieve thermal stability in a winter cluster.”
“The temperature at the center, the periphery and the entrance of a honey bee colony was continuously determined during the summer season and the broodless time in winter. During the summer season the temperature in the brood nest averages 35.5°C (95.9°F) with brief excursions up to 37.0°C (98.6°F) and down to 33.8°C (98.8°F). Increasing environmental temperatures resulted in linear increases in the temperature of the hive entrance, its periphery, and its center. The temperature in the center of an overwintering cluster is maintained at an average value of 21.3°C (70.3°F) (min. 12.0°C (53.6°F), max. 33.5°C (92.3°F). With rising ambient temperatures, the central temperature of a winter cluster drops whereas the peripheral temperature increases slightly. With decreasing external temperatures, the peripheral temperature is lowered by a small amount while the cluster’s center temperature is raised.
Linear relationships are observed between the central and the ambient temperature and between the central temperature and the temperature difference of the peripheral and the ambient temperatures. The slopes point to two minimum threshold values for the central (15°C, 59.0°F) and the peripheral temperature (5°C, 41.0°F) which should not be transgressed in an overwintering cluster. Microcalorimetric determinations of the heat production were performed on the three castes of the honey bee: workers, drones and queens of different ages. Among these groups single adult workers showed the highest heat production rates (209 mW.g-1) (mW=milliwatt) with only neglectable fluctuations in heat production rate. Juvenile workers exhibited a mean heat production rate of 142 mW.g-1. The rate of heat production of adult workers is strongly dependent upon the number of bees together in a group. With more than 10 individuals, weight-specific heat dissipation remains constant with increasing group sizes at a level approximately 1/17 that of an isolated bee.
Differences are seen between the rates of virgin (117 mW.g-1) and laying (102 mW.g-1) queens. Laying queens showed less thermal fluctuations than virgin queens. High fluctuations in heat production rates were observed for drones. In both drone groups (fertile, juvenile) phases of high and extremely low activity succeed one another. The heat production of juvenile drones was 68 mW.g-1, that of fertile drones 184 mW.g-1 due to stronger locomotory activities (Fahrenholz et al. 1989).”
“Sumpter and Broomhead (2000) developed a model to investigate the movement of individuals in thermoregulating honey bee clusters. Thermoregulation in over-wintering clusters is thought to be the result of individual bees attempting to regulate their own body temperatures. At ambient temperatures above 0°C (32°F), a clustering bee will move relative to its neighbors so as to put its local temperature within some ideal range. Computer simulation of this model demonstrates qualitative behavior which agrees with that of real honey bee clusters. In particular, they observed the formation of both disc- and ring-like cluster shapes. The simulation also suggests that at lower ambient temperatures, clusters do not always have a stable shape but can oscillate between insulating rings of different sizes and densities.
The computer model they developed is based on the following assumptions about the behavior of individual bees: 1. Each bee bases her behavior exclusively on her local temperature. 2. Bees have a preferred range of temperatures. Inside this range a bee moves randomly. When she is outside this range she will move in the appropriate direction along the temperature gradient. 3. Below a lower threshold temperature a bee will go into a “chill coma” and will be unable to move. 4. A bee’s heat production is based on her metabolic rate which is an increasing function of temperature. Bees in a coma generate no heat.”
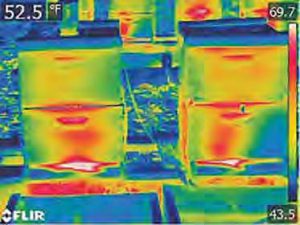
Infrared heat of colony
“A thermal imaging method was used to monitor thermal processes in the inter-comb bee clusters and temperature of different body parts during the wintering period. The temperature of different body parts was found to depend on the localization of bees in the nest and the external temperature. The dependence of the thermoregulatory activity of bees on the external temperature fluctuations decreased during wintering. The trends of distribution of thermal fields in clusters of wintering bees were revealed (Eskov and Toboev 2011).”
“Winter cluster volume and surface area were measured over a range of ambient temperatures (Ta) in 3 honey bee colonies representative of small, medium and large populations from 29 November 1984 to 21 March 1985 (Severson and Erickson 1990). Changes in these parameters were correlated with changes in Ta and the observed response to Ta was independent of population size. They observed decreases of about 55% in cluster volume and 40% in cluster surface area as the Ta decreased from 4°C (39.2°F) to -23°C (-9.4°F).”
“Experiments conducted over three winters have revealed a metabolism controlling function of bee-induced hypoxia in the winter cluster. Permanent low oxygen levels around 15% were found in its core. This hypoxia was actively controlled, probably via indirect mechanisms. Varying ambient oxygen levels demonstrated a causal relationship between lowered oxygen and reduced metabolic rate (MR). Under deeper ambient hypoxia the bees switched to ultra-low metabolic rate (ULMR), optional-occasional at 15% oxygen, obligatory at 7.5% oxygen. This dormancy status resembled deep diapause in insects. It stayed reversible after at least several days and was terminated under normal oxygen at 15°C (59°F). Reduced MR via core-hypoxia is essential in water conserving thermoregulation of the winter cluster. It allows bees to reconcile warm wintering in alert state—for defense of stores—with energy saving and longevity (Van Nerum and Buelens 1997).”
“During the broodless period in winter, the pollen content of the gastrointestinal tract, the degree of pollen digestion and the proteolytic activity in the midgut were investigated in bees from the margin and from the center of winter clusters of two colonies in Austria. In addition, the movement of bees within the winter cluster was examined. There was no difference in pollen content and proteolytic activity between bees from the center or margin of the cluster, nor did the two groups show a preference for staying at the center or on the margin of the winter cluster. Compared to 8-9-day-old bees in summer, the amount of pollen in the midguts was smaller by a factor of 100-1000, but the degree of pollen digestion in the midgut and the rectum was significantly greater; the proteolytic activity in the midgut was approximately a quarter. The more efficient utilization in spite of lower proteolytic activity might be due to pollen staying longer in the midgut. Foragers in summer also consume only minimal amounts of pollen but have a smaller degree of utilization than winter bees. The reduced pollen consumption rate, and efficient utilization in spite of lower proteolytic activity are useful adaptations to the reduced availability of pollen and reduced protein metabolism which bees experience during the winter (Crailsheim et al. 1993).”
“Microbial symbionts inhabiting the honey bee gut (i.e., gut microbiota) are essential for food digestion, immunity and gut protection of their host. The taxonomic composition of the gut microbiota is dynamic throughout the honey bee life cycle and the foraging season. However, it remains unclear how drastic changes occurring in winter, such as food shortage and cold weather, impact gut microbiota dynamics. Bleau et al. (2020) characterized the gut microbiota of the honey bee during the overwintering period in a northern temperate climate in Canada. The microbiota of nine colonies was characterized by metataxonomy of 16S rDNA between September 2017 and June 2018. Overall, the results showed that microbiota taxonomic composition experienced major compositional shifts in fall and spring. From September to November, Enterobacteriaceae decreased, while Neisseriaceae increased. From April to June, Orbaceae increased, whereas Rhizobiaceae nearly disappeared. Bacterial diversity of the gut microbiota decreased drastically before and after overwintering, but it remained stable during winter. They concluded that the gut microbiota is likely to be impacted by the important meteorological and dietary changes that take place before and after the overwintering period.”
“In winter, honey bees thermoregulate their hives to survive cold temperatures and maintain their physiological activity, without becoming completely dormant. During this time, nurses and foragers are not distinguishable. In late winter or early spring, as the brood rearing re-initiates, the division of labor resumes among the workers born in the fall. To understand the overall physiological changes of honey bee workers from late winter (end of over-wintering) to early spring (beginning of brood rearing), Lee and Kim (2017) collected honey bees in January and February and compared their protein expression profiles. Among the 50 and 85 proteins showing greater than two-fold differences in expression levels in the head and abdomen, respectively, 20 proteins with relatively large differences in expression level between the months were selected and identified. Most proteins were more abundantly expressed in January than February and were mainly involved in nutrient storage, energy metabolism, and biosynthesis pathways in both the head and abdomen. This finding suggested that overwintering bees require large energy storage and metabolize stored nutrition to generate high cellular energy for thermoregulation of their hive without diapause and/or to prepare for the initiation of brood rearing in January.”
“Thermoregulation is crucial for colony survival in temperate regions, but possible interference by parasites is currently unknown. The small hive beetle (Aethina tumida) and the ectoparasitic mite Varroa destructor are honey bee parasites that overwinter in host colonies. The efficiency of thermoregulation might thus be affected in infested host winter clusters, due to altered worker activity. Schӓfer et al. (2011) showed for the first time that parasites can alter honey bee thermoregulation. Moreover, the data suggested that only combined infestations with V. destructor and A. tumida resulted in higher thermal maxima in the winter clusters, whereas infestations with one parasite alone had no significant effect compared with the controls. Due to the ubiquitous mite V. destructor combined infestations with parasites or combined infections with pathogens are almost inevitable. Therefore, their data indicated that an altered thermoregulation due to multiple infestations might be another widespread factor contributing to winter losses of colonies.”
“Extreme winter losses of honey bee colonies are a major threat to beekeeping but the combinations of factors underlying colony loss remain debatable. Desai and Currie (2016) monitored colonies in two environments (colonies wintered indoors or outdoors) and characterized the effects of two parasitic mites, seven viruses, and Nosema on colony mortality and population loss over winter. Samples were collected from two locations within hives in fall, mid-winter, and spring of 2009/2010. Although fall parasite and pathogen loads were similar in outdoor and indoor-wintered colonies, the outdoor-wintered colonies had greater relative reductions in bee population score over winter. Seasonal patterns in deformed wing virus (DWV), black queen cell virus (BQCV), and Nosema level also differed with the wintering environment. DWV and Nosema levels decreased over winter for indoor-wintered colonies but BQCV did not. Both BQCV and Nosema concentration increased over winter in outdoor-wintered colonies. The mean abundance of Varroa decreased and concentration of Sacbrood virus (SBV), Kashmir bee virus (KBV), and Chronic bee paralysis (CBPV) increased over-winter but seasonal patterns were not affected by wintering method. For most viruses, either entrance or brood area samples were reasonable predictors of colony virus load but there were significant season*sample location interactions for Nosema and BQCV, indicating that care must be taken when selecting samples from a single location. For Nosema spp., the fall entrance samples were better predictors of future infestation levels than were fall brood area samples. For indoor-wintered colonies, Israeli acute paralysis virus IAPV concentration was negatively correlated with spring population size. For outdoor-wintered hives, spring Varroa abundance and DWV concentration were positively correlated with bee loss and negatively correlated with spring population size. Multivariate analyses for fall collected samples indicated higher DWV was associated with colony death as did high SBV for spring-collected samples.









